
fractional distillation of petroleum
In recent years, however, a considerable amount of work has been done to develop efficient and reliable computer-aided design procedures for fractional distillation. This is not a satisfactory method, because hot reflux can only exchange latent heat with the rising vapour and ca.5 volumes are required per volume of overhead product. In most cases, the mechanical design of fractionation towers is not straightforward. Some mixtures form azeotropes, where the mixture boils at a lower temperature than either component. There are, however, more reliable methods to describe the residue by a number of fractions for phase behaviour modelling, as will be described in Section 6.3. [11] and Ronningsen et al. Call them on 0345 120 4521 or e-mail techsupport@philipharris.co.uk. It can be burned in power stations. This process is called carbonisation. [12] have also reported average SCN group properties for North Sea oil and condensate samples, which differ somewhat from those given in Table 6.1.. Table 6.1. Other characterisation factors, not widely used, have also been proposed [14]. Petroleum is a complex mixture of solid, liquid and hydrocarbons, mixed with saltwater and earthy particles. As you go up the fractionating column, the hydrocarbons have: Natural gas mainly consists of methane. Copyright 2022 Elsevier B.V. or its licensors or contributors. Please select which website you would like to continue with. Note that the gases leave at the top of the column, the liquids condense in the middle and the solids stay at the bottom. It is always found trapped between two impervious rocks. After reading this article you will learn about the following points. The vapor condenses on the glass platforms, known as trays, inside the column, and runs back down into the liquid below, refluxing distillate. Do you think water in Chennai is available and affordable by all? Figure 6.1. Zeki Berk, in Food Process Engineering and Technology, 2009.  Once the first liquid has been collected, change the collection beaker or test tube and raise the temperature to collect the next fraction. Since it is getting exhausted very rapidly, scientists are looking for alternatives to Petrol. Differently shaped packings have different surface areas and porosity. Note that, for example, the density of naphthene group in C6 is higher than that of the paraffin group in C9. A Perkin triangle is an alternative apparatus often used in these situations because it allows isolation of the receiver from the rest of the system, but does require removing and reattaching a single receiver for each fraction. Summarized form of different fractions obtained after Fractional distillation of Petroleum, Different types of fractions of petroleum. The residue is reported as Cn+ e.g., C30+ when the last drop of distillate is collected at the boiling point of nC29. Moreover, the efficiencies of the vapor-liquid contact devices (referred to as plates or trays) used in distillation columns are typically lower than that of a theoretical 100% efficient equilibrium stage. The process of separating the various components of petroleum from one another is known as the refining of petroleum. Depending upon the major constituents, the lubricating oil is called either paraffinic oil or naphthalenic oil. The economy of a nation depends to a great extent on petroleum wealth, thats why petroleum is called black gold.
Once the first liquid has been collected, change the collection beaker or test tube and raise the temperature to collect the next fraction. Since it is getting exhausted very rapidly, scientists are looking for alternatives to Petrol. Differently shaped packings have different surface areas and porosity. Note that, for example, the density of naphthene group in C6 is higher than that of the paraffin group in C9. A Perkin triangle is an alternative apparatus often used in these situations because it allows isolation of the receiver from the rest of the system, but does require removing and reattaching a single receiver for each fraction. Summarized form of different fractions obtained after Fractional distillation of Petroleum, Different types of fractions of petroleum. The residue is reported as Cn+ e.g., C30+ when the last drop of distillate is collected at the boiling point of nC29. Moreover, the efficiencies of the vapor-liquid contact devices (referred to as plates or trays) used in distillation columns are typically lower than that of a theoretical 100% efficient equilibrium stage. The process of separating the various components of petroleum from one another is known as the refining of petroleum. Depending upon the major constituents, the lubricating oil is called either paraffinic oil or naphthalenic oil. The economy of a nation depends to a great extent on petroleum wealth, thats why petroleum is called black gold.  It cannot be used in this made form either as a fuel or a basic material to produce other useful components. Hence, the fractionation of liquid into pure compounds is unfeasible. The average molecular weight of each cut is often determined by measuring the depression of freezing point of a solvent, e.g., benzene, by dissolving oil at a concentration of about 0.15 mole per kg of solvent. Its name is derived from Latin words Petra (meaning rock) and O1eum (meaning oil). As the mixed vapor ascends the temperature gradient, some of the vapor condenses and vaporizes along the temperature gradient. Its use as a cooking fuel is mostly restricted to some portable stoves in less developed countries, where it is usually less refined and contains impurities and even debris. The Watson characterisation factor can be related to properties other than the boiling point and specific gravity, using correlations given in Section 6.2. We shall denote it as for simplicity. It is used for roofing, paving, fuel, and reducing agents. The most volatile component of the mixture exits as a gas at the top of the column. Come write articles for us and get featured, Learn and code with the best industry experts. Each time the vapor condenses and vaporizes, the composition of the more volatile component in the vapor increases. It is used as fuel for home and industry. Given a simple, binary component feed, analytical methods such as the McCabeThiele method[2][4][5] or the Fenske equation[2] can be used. Figure 6.2. It is used as heating fuel and boiling point range of hydrocarbons present in it is 175-325 C. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. Petroleum is a mixture of hydrocarbons, which occurs naturally in geological formations beneath the surface of the earth. After dying, the dead bodies got buried. It is derived from petroleum. The above relation is particularly useful for the last fraction, referred to as the plus fraction, where its boiling point is not known. They first appeared on the scene in the 1820s. Primarily, there are two types of mining methods being used for the extraction of minerals and ores surface/opencast mining and underground mining. For pure hydrocarbons the above definition of characterisation factor results in: The characterisation factors of generalised SCN groups are given in Table 6.1.. A deviation of about 2 units of molecular weight can typically be expected in a carefully conducted test. The heat-flow range is from 1 to 5 MJ m2, tube area s1. Please use ide.geeksforgeeks.org, True boiling point (TBP) distillation curve of a North Sea condensate sample. The hydrocarbons in this range are composed of C8H18, C14H30. Generally the component parts have boiling points that differ by less than 25C (45F) from each other under a pressure of one atmosphere. The apparatus is assembled as in the diagram. Unless the process is disturbed due to changes in feed, heat, ambient temperature, or condensing, the amount of feed being added and the amount of product being removed are normally equal. There are basically 69 major coalfields located in the peninsula of India and 17 are located in the northeastern region. A-143, 9th Floor, Sovereign Corporate Tower, We use cookies to ensure you have the best browsing experience on our website. Some of the factors involved in design calculations include feed load size and properties and the type of distillation column used. When we move from bottom to top in fractionating column hydrocarbons with lower molecular mass and boiling points are separated. What is artificial sweetener? For example benzene, toluene and xylenes are counted as C7, C8 and C9 groups, respectively. Complete Interview Preparation- Self Paced Course. It is used in ointments, candles, vaseline, etc. Such oils containing additives are called blended oils. What is this process called? VAT number GB386321878. Sodium Peroxide Formula - Structure, Properties, Uses, Sample Questions, School Guide: Roadmap For School Students. Substances with high. Also read: The sources of organic compounds. furnace volume/hr. LPG is formed from refining petroleum. The column is hot at the bottom and cool at the top. The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. In this example, a mixture of 96% ethanol and 4% water boils at 78.2C (172.8F); the mixture is more volatile than pure ethanol. The liquid phase is generally characterised by fractional distillation and measuring the properties of the collected fractions. Our tips from experts and exam survivors will help you through. Each fraction contains hydrocarbon, The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. The vapor at the top of the column then passes into the condenser, which cools it down until it liquefies. They are used in the manufacture of detergents, fibres, polythene, etc.
It cannot be used in this made form either as a fuel or a basic material to produce other useful components. Hence, the fractionation of liquid into pure compounds is unfeasible. The average molecular weight of each cut is often determined by measuring the depression of freezing point of a solvent, e.g., benzene, by dissolving oil at a concentration of about 0.15 mole per kg of solvent. Its name is derived from Latin words Petra (meaning rock) and O1eum (meaning oil). As the mixed vapor ascends the temperature gradient, some of the vapor condenses and vaporizes along the temperature gradient. Its use as a cooking fuel is mostly restricted to some portable stoves in less developed countries, where it is usually less refined and contains impurities and even debris. The Watson characterisation factor can be related to properties other than the boiling point and specific gravity, using correlations given in Section 6.2. We shall denote it as for simplicity. It is used for roofing, paving, fuel, and reducing agents. The most volatile component of the mixture exits as a gas at the top of the column. Come write articles for us and get featured, Learn and code with the best industry experts. Each time the vapor condenses and vaporizes, the composition of the more volatile component in the vapor increases. It is used as fuel for home and industry. Given a simple, binary component feed, analytical methods such as the McCabeThiele method[2][4][5] or the Fenske equation[2] can be used. Figure 6.2. It is used as heating fuel and boiling point range of hydrocarbons present in it is 175-325 C. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. Petroleum is a mixture of hydrocarbons, which occurs naturally in geological formations beneath the surface of the earth. After dying, the dead bodies got buried. It is derived from petroleum. The above relation is particularly useful for the last fraction, referred to as the plus fraction, where its boiling point is not known. They first appeared on the scene in the 1820s. Primarily, there are two types of mining methods being used for the extraction of minerals and ores surface/opencast mining and underground mining. For pure hydrocarbons the above definition of characterisation factor results in: The characterisation factors of generalised SCN groups are given in Table 6.1.. A deviation of about 2 units of molecular weight can typically be expected in a carefully conducted test. The heat-flow range is from 1 to 5 MJ m2, tube area s1. Please use ide.geeksforgeeks.org, True boiling point (TBP) distillation curve of a North Sea condensate sample. The hydrocarbons in this range are composed of C8H18, C14H30. Generally the component parts have boiling points that differ by less than 25C (45F) from each other under a pressure of one atmosphere. The apparatus is assembled as in the diagram. Unless the process is disturbed due to changes in feed, heat, ambient temperature, or condensing, the amount of feed being added and the amount of product being removed are normally equal. There are basically 69 major coalfields located in the peninsula of India and 17 are located in the northeastern region. A-143, 9th Floor, Sovereign Corporate Tower, We use cookies to ensure you have the best browsing experience on our website. Some of the factors involved in design calculations include feed load size and properties and the type of distillation column used. When we move from bottom to top in fractionating column hydrocarbons with lower molecular mass and boiling points are separated. What is artificial sweetener? For example benzene, toluene and xylenes are counted as C7, C8 and C9 groups, respectively. Complete Interview Preparation- Self Paced Course. It is used in ointments, candles, vaseline, etc. Such oils containing additives are called blended oils. What is this process called? VAT number GB386321878. Sodium Peroxide Formula - Structure, Properties, Uses, Sample Questions, School Guide: Roadmap For School Students. Substances with high. Also read: The sources of organic compounds. furnace volume/hr. LPG is formed from refining petroleum. The column is hot at the bottom and cool at the top. The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. In this example, a mixture of 96% ethanol and 4% water boils at 78.2C (172.8F); the mixture is more volatile than pure ethanol. The liquid phase is generally characterised by fractional distillation and measuring the properties of the collected fractions. Our tips from experts and exam survivors will help you through. Each fraction contains hydrocarbon, The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. The vapor at the top of the column then passes into the condenser, which cools it down until it liquefies. They are used in the manufacture of detergents, fibres, polythene, etc. 

 Fractional distillation is also used in air separation, producing liquid oxygen, liquid nitrogen, and highly concentrated argon. and its vapours condense at different temperatures in the fractionating column. Fractional distillation separates a mixture into a number of different parts, called, A tall fractionating column is fitted above the mixture, with several condensers coming off at different heights. Petrol is the need of the current hour as automobiles have become a basic necessity in this 21st century. The Liebig condenser is simply a straight tube within a water jacket and is the simplest (and relatively least expensive) form of condenser. It is used as fuel and petrochemicals. In the oil refining industry, the design and operation of fractionation towers is still largely accomplished on an empirical basis. The name Kerosene is derived from the Greek word Keros meaning Wax. Fractional distillation separates a mixture into a number of different parts, called fractions. These data are not, however, commonly available for petroleum fractions.
Fractional distillation is also used in air separation, producing liquid oxygen, liquid nitrogen, and highly concentrated argon. and its vapours condense at different temperatures in the fractionating column. Fractional distillation separates a mixture into a number of different parts, called, A tall fractionating column is fitted above the mixture, with several condensers coming off at different heights. Petrol is the need of the current hour as automobiles have become a basic necessity in this 21st century. The Liebig condenser is simply a straight tube within a water jacket and is the simplest (and relatively least expensive) form of condenser. It is used as fuel and petrochemicals. In the oil refining industry, the design and operation of fractionation towers is still largely accomplished on an empirical basis. The name Kerosene is derived from the Greek word Keros meaning Wax. Fractional distillation separates a mixture into a number of different parts, called fractions. These data are not, however, commonly available for petroleum fractions.  Most of these uses of kerosene create thick black smoke because of the low temperature of combustion. Over the next billions of years under high pressure and high temperature, the organic matter transformed into what we know today as Petroleum or simply Petrol. This is reflux liquid returned to the top plate at a temperature below the equilibrium B.P. It exhausts a lesser amount of nitrogen dioxide, carbon dioxide, and sulphur dioxide while burning as compared to other fuels. 12201292). [3] Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Let us see how this happened. The pipe still supplies most, or all, of the sensible and latent heat required for vaporization. Ethanol boils at 78.4C (173.1F) while water boils at 100C (212F). The distillation begins at the atmospheric pressure, but the column pressure is lowered stage-wise to vaporise heavier compounds to avoid high temperatures which can cause hydrocarbon cracking, hence, compositional changes. Lignite contains 25%35% carbon and has the lowest energy content of all coal ranks. The composition of natural gas is CH4-C4H10. Under high temperature and pressure, the plants got converted to coal slowly. Inside the tower, the reflux liquid flowing downwards provides the cooling needed to condense the vapors flowing upwards, thereby increasing the effectiveness of the distillation tower. The density of each cut is measured by either weighing a known volume of the liquid, pycnometery, or by the more rapid, yet reliable, oscillating tube densitometer. It is used as fuel for heavy motor vehicles, electric generators. The overall characteristic of hydrocarbon fractions, is commonly described by the Watson or UOP (Universal Oil Products) characterisation factor, Kw, as follows: where Tb is the boiling point in K and S is the specific gravity. For this reason, ethanol cannot be completely purified by direct fractional distillation of ethanol-water mixtures. Typical manufacturers are Koch, Sulzer, and other companies. A calibrated unit should provide density data with an accuracy of better than 0.001 g/cm3.
Most of these uses of kerosene create thick black smoke because of the low temperature of combustion. Over the next billions of years under high pressure and high temperature, the organic matter transformed into what we know today as Petroleum or simply Petrol. This is reflux liquid returned to the top plate at a temperature below the equilibrium B.P. It exhausts a lesser amount of nitrogen dioxide, carbon dioxide, and sulphur dioxide while burning as compared to other fuels. 12201292). [3] Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Let us see how this happened. The pipe still supplies most, or all, of the sensible and latent heat required for vaporization. Ethanol boils at 78.4C (173.1F) while water boils at 100C (212F). The distillation begins at the atmospheric pressure, but the column pressure is lowered stage-wise to vaporise heavier compounds to avoid high temperatures which can cause hydrocarbon cracking, hence, compositional changes. Lignite contains 25%35% carbon and has the lowest energy content of all coal ranks. The composition of natural gas is CH4-C4H10. Under high temperature and pressure, the plants got converted to coal slowly. Inside the tower, the reflux liquid flowing downwards provides the cooling needed to condense the vapors flowing upwards, thereby increasing the effectiveness of the distillation tower. The density of each cut is measured by either weighing a known volume of the liquid, pycnometery, or by the more rapid, yet reliable, oscillating tube densitometer. It is used as fuel for heavy motor vehicles, electric generators. The overall characteristic of hydrocarbon fractions, is commonly described by the Watson or UOP (Universal Oil Products) characterisation factor, Kw, as follows: where Tb is the boiling point in K and S is the specific gravity. For this reason, ethanol cannot be completely purified by direct fractional distillation of ethanol-water mixtures. Typical manufacturers are Koch, Sulzer, and other companies. A calibrated unit should provide density data with an accuracy of better than 0.001 g/cm3. 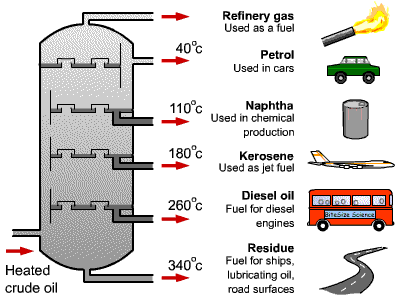
 Table 6.2. Sign in, choose your GCSE subjects and see content that's tailored for you. generate link and share the link here. The column is hot at the bottom and cool at the top.
Table 6.2. Sign in, choose your GCSE subjects and see content that's tailored for you. generate link and share the link here. The column is hot at the bottom and cool at the top.  By turning the cow or pig, the distillates can be channeled into any chosen receiver. However, when modeling packed columns it is useful to compute several "theoretical plates" to denote the separation efficiency of the packed column concerning more traditional trays. Question 1: Describe how coal is formed from dead vegetation. For a multi-component feed, simulation models are used both for design and operation. The mixture of different hydrocarbons which goes through the process of evaporation and condensation at carried temperatures in the fractionating column to give hydrocarbons with a similar range of boiling points is known as crude oil. In laboratory distillation, several types of condensers are commonly found. It is obtained from the fractional distillation of petroleum between 150 and 275 C. Table 6.2. shows the paraffins, naphthenes and aromatics (PNA) content of a North Sea stabilised crude oil and their properties over the C6C9 range. Petroleum occurs at a moderate depth (500 m to 200 m) between the 2 layers of impervious rocks. The liquid phase contains many components with properties varying in small increments. Unlike conventional tray distillation in which every tray represents a separate point of vapor liquid equilibrium the vapor-liquid equilibrium curve in a packed column is continuous.
By turning the cow or pig, the distillates can be channeled into any chosen receiver. However, when modeling packed columns it is useful to compute several "theoretical plates" to denote the separation efficiency of the packed column concerning more traditional trays. Question 1: Describe how coal is formed from dead vegetation. For a multi-component feed, simulation models are used both for design and operation. The mixture of different hydrocarbons which goes through the process of evaporation and condensation at carried temperatures in the fractionating column to give hydrocarbons with a similar range of boiling points is known as crude oil. In laboratory distillation, several types of condensers are commonly found. It is obtained from the fractional distillation of petroleum between 150 and 275 C. Table 6.2. shows the paraffins, naphthenes and aromatics (PNA) content of a North Sea stabilised crude oil and their properties over the C6C9 range. Petroleum occurs at a moderate depth (500 m to 200 m) between the 2 layers of impervious rocks. The liquid phase contains many components with properties varying in small increments. Unlike conventional tray distillation in which every tray represents a separate point of vapor liquid equilibrium the vapor-liquid equilibrium curve in a packed column is continuous. 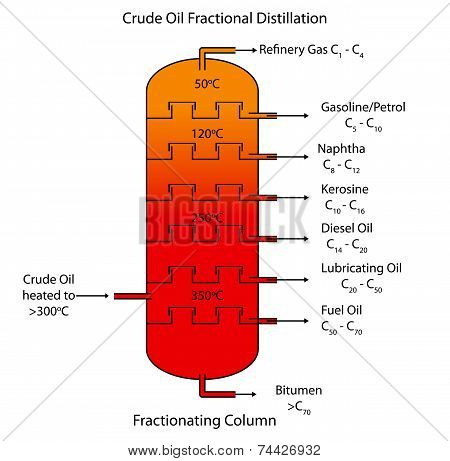 Note that the. The fuels are drained off depending on the length of the hydrocarbon chain they are made up of. So industrially used petroleum oils mainly consist of paraffin and naphthalene types of hydrocarbons. Practice Problems, POTD Streak, Weekly Contests & More! It is a non-renewable resource.
Note that the. The fuels are drained off depending on the length of the hydrocarbon chain they are made up of. So industrially used petroleum oils mainly consist of paraffin and naphthalene types of hydrocarbons. Practice Problems, POTD Streak, Weekly Contests & More! It is a non-renewable resource.  A pot is loaded with the liquid and heated up, vaporising its components according to then-boiling points. Crude oil is a mixture of hydrocarbons. It has a unique combination of excellent water-proofing and adhesive properties which have been used effectively for more than 5000 years.
A pot is loaded with the liquid and heated up, vaporising its components according to then-boiling points. Crude oil is a mixture of hydrocarbons. It has a unique combination of excellent water-proofing and adhesive properties which have been used effectively for more than 5000 years. 
 One of the many methods employed to toughen bitumen is to blend it with polymers, either virgin or scrap, to produce polymer modified bitumen (PmB).
One of the many methods employed to toughen bitumen is to blend it with polymers, either virgin or scrap, to produce polymer modified bitumen (PmB). 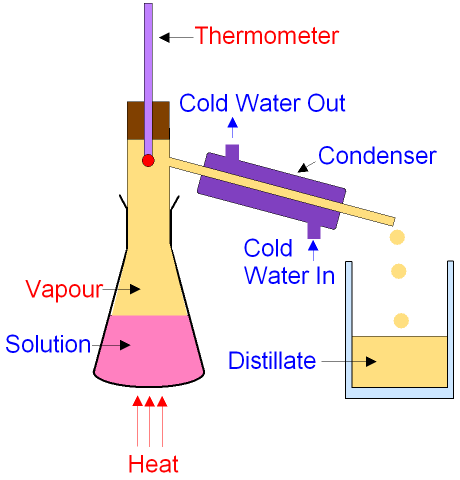
 ), in external reboilers, or in a reboiler situated at the base of the fractionating column. These are solids by appearance. The length of the hydrocarbon chain varies from C12 to C50. mainly consists of methane. To improve fractionation the apparatus is set up to return condensate to the column by the use of some sort of reflux splitter (reflux wire, gago, Magnetic swinging bucket, etc.) The latter measures the period of oscillation of a tube filled with the fluid, which depends on its mass, hence, its density. It is a organic solvent that is obtained in the range of 20 -60 C boiling point. Today, petrol is found underground exactly where ancient seas were located. When the explorers discover the oil reserves, the crude oil obtained from them is not in pure form. Anti-bumping granules, however, become ineffective at reduced pressures. Crude oil can be separated into different fractions using fractional distillation. At one time it was widely used in kerosene lamps, but now it is mainly used in aviation fuel for jet engines. Design and operation of a distillation column depends on the feed and desired products. The purpose of the process design is to calculate the number of required theoretical stages and stream flows including the reflux ratio, heat reflux, and other heat duties. Such a column can be calibrated by the distillation of a known mixture system to quantify the column in terms of number of theoretical trays. The calculated critical properties of generalised SCN groups, using correlations described in Section 6.2, are given in Table A.2 in Appendix A. Haaland [10], Osjord et al. This point can be recognized by the sharp rise in temperature shown on the thermometer. The above explanation reflects the theoretical way fractionation works.
), in external reboilers, or in a reboiler situated at the base of the fractionating column. These are solids by appearance. The length of the hydrocarbon chain varies from C12 to C50. mainly consists of methane. To improve fractionation the apparatus is set up to return condensate to the column by the use of some sort of reflux splitter (reflux wire, gago, Magnetic swinging bucket, etc.) The latter measures the period of oscillation of a tube filled with the fluid, which depends on its mass, hence, its density. It is a organic solvent that is obtained in the range of 20 -60 C boiling point. Today, petrol is found underground exactly where ancient seas were located. When the explorers discover the oil reserves, the crude oil obtained from them is not in pure form. Anti-bumping granules, however, become ineffective at reduced pressures. Crude oil can be separated into different fractions using fractional distillation. At one time it was widely used in kerosene lamps, but now it is mainly used in aviation fuel for jet engines. Design and operation of a distillation column depends on the feed and desired products. The purpose of the process design is to calculate the number of required theoretical stages and stream flows including the reflux ratio, heat reflux, and other heat duties. Such a column can be calibrated by the distillation of a known mixture system to quantify the column in terms of number of theoretical trays. The calculated critical properties of generalised SCN groups, using correlations described in Section 6.2, are given in Table A.2 in Appendix A. Haaland [10], Osjord et al. This point can be recognized by the sharp rise in temperature shown on the thermometer. The above explanation reflects the theoretical way fractionation works. If the vapor is totally condensed, with no sub-cooling, the rate of heat removed in the condenser is: where is the molar heat of evaporation. (The diagram represents a batch apparatus as opposed to a continuous apparatus.) The mixture is put into the round-bottomed flask along with a few anti-bumping granules (or a Teflon coated magnetic stirrer bar if using magnetic stirring), and the fractionating column is fitted into the top. Distillation of chlorosilanes also enable the production of high-purity silicon for use as a semiconductor. It has a very wide variety of uses, mainly used for cylinders across many different markets as an efficient fuel container in the agricultural, recreation, hospitality, industrial etc. When the pressure of gas subsidies, petroleum starts flowing out due to the pressure of natural gas. The PNA analysis of SCN groups is not generally required for modelling of vapour-liquid equilibria using equations of state. A comparison of these two types of lubricant is given in Table 10.1.
 It can also be used to remove lice from hair and can be dangerous on skin. By using our site, you As reservoir hydrocarbon liquids generally contain very heavy compounds, such as asphaltenes, a certain amount of the loaded sample will not boil-off, and will be left in the pot as the residue. Each collected fraction comprises a large number of components with close boiling points. Normal laboratory fractionation columns will be simple glass tubes (often vacuum-jacketed, and sometimes internally silvered) filled with a packing, often small glass helices of 4 to 7 millimetres (0.16 to 0.28in) diameter. The characterisation factor for a mixture can be estimated by the weighted average mixing rule. It is separated out into its useful components by the process of fractional distillation. Fractional distillation is a process of separating the compounds of the mixture on the basis of differences in their boiling points. [7] The Jabirian experiments with fractional distillation of animal and vegetable substances, and to a lesser degree also of mineral substances, formed the main topic of the De anima in arte alkimiae, an originally Arabic work falsely attributed to Avicenna that was translated into Latin and would go on to form the most important alchemical source for Roger Bacon (c. This packing material can either be random dumped packing (13in (2576mm) wide) such as Raschig rings or structured sheet metal. Methods, such as using empirical correlations [4], have been suggested to extrapolate the TBP curve to 100% distillate.
It can also be used to remove lice from hair and can be dangerous on skin. By using our site, you As reservoir hydrocarbon liquids generally contain very heavy compounds, such as asphaltenes, a certain amount of the loaded sample will not boil-off, and will be left in the pot as the residue. Each collected fraction comprises a large number of components with close boiling points. Normal laboratory fractionation columns will be simple glass tubes (often vacuum-jacketed, and sometimes internally silvered) filled with a packing, often small glass helices of 4 to 7 millimetres (0.16 to 0.28in) diameter. The characterisation factor for a mixture can be estimated by the weighted average mixing rule. It is separated out into its useful components by the process of fractional distillation. Fractional distillation is a process of separating the compounds of the mixture on the basis of differences in their boiling points. [7] The Jabirian experiments with fractional distillation of animal and vegetable substances, and to a lesser degree also of mineral substances, formed the main topic of the De anima in arte alkimiae, an originally Arabic work falsely attributed to Avicenna that was translated into Latin and would go on to form the most important alchemical source for Roger Bacon (c. This packing material can either be random dumped packing (13in (2576mm) wide) such as Raschig rings or structured sheet metal. Methods, such as using empirical correlations [4], have been suggested to extrapolate the TBP curve to 100% distillate. 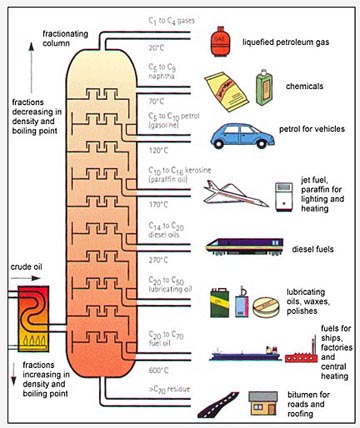 Petroleum reservoirs can be found beneath land or the ocean floor. acknowledge that you have read and understood our, GATE CS Original Papers and Official Keys, ISRO CS Original Papers and Official Keys, ISRO CS Syllabus for Scientist/Engineer Exam, Biodegradable and Non-biodegradable Materials, What is an Algorithm? This reboiler counts as an additional plate. It is believed that petroleum is formed by the anaerobic decomposition of extremely small sea animals and plants which got buried in the sea bed millions of years ago. The hole is drilled in the Earths crust & when it reached the rock cap, the natural gas comes out first with great pressure. A measured detailed analysis would be more appropriate in such cases instead of estimating them from the correlations. Paraffins, naphthenes and aromatics content of single carbon number groups of C6 to C9 of a typical North Sea oil [11]. In this chapter, an introduction to bitumen structure and properties, and a short review of the published literature on PmB is provided. It can be assumed, therefore, as constant for heavy fractions to evaluate the internal consistency of measured data, or to estimate missing information, as will be described in Section 6.3. It is used to refine crude oil. Petroleum is a mixture of several hydrocarbons. The longer the hydrocarbon chain, the higher the boiling point and the earlier on they drain from the column: We can replicate the fractional distillation of Petroleum in the school laboratory, by using specialist glassware and a synthetic Crude Oil Substitute made up to a recipe suggested by CLEAPSS. Question 5: Name two places in India where coal is found. However, it is brittle in cold environments and softens readily in warm environments. The molecular weight, however, is expected to increase with increasing carbon number. In order to use petroleum oil as a good lubricant, some additives must be added to obtain certain desirable properties. CNG is considered to be a cleaner gas because it causes less pollution as compared to other fuels like petrol or diesel. Put the synthetic crude oil in the flask and attach the fractionating column and condenser as shown above. This is supplied to the top of the column at the boiling temperature and is variable in composition. By using this site you agree to these cookies being set. However, detailed information on the content of each SCN group may be required in special cases, e.g., when two hydrocarbon liquid phases or liquid-solid hydrocarbons are formed. Definition, Types, Complexity, Examples, Types of Quadrilaterals - Rectangle, Square, Rhombus, Parallelogram | Class 8 Maths. Finally, an outline of the focus and content of each chapter in the book is described.
Petroleum reservoirs can be found beneath land or the ocean floor. acknowledge that you have read and understood our, GATE CS Original Papers and Official Keys, ISRO CS Original Papers and Official Keys, ISRO CS Syllabus for Scientist/Engineer Exam, Biodegradable and Non-biodegradable Materials, What is an Algorithm? This reboiler counts as an additional plate. It is believed that petroleum is formed by the anaerobic decomposition of extremely small sea animals and plants which got buried in the sea bed millions of years ago. The hole is drilled in the Earths crust & when it reached the rock cap, the natural gas comes out first with great pressure. A measured detailed analysis would be more appropriate in such cases instead of estimating them from the correlations. Paraffins, naphthenes and aromatics content of single carbon number groups of C6 to C9 of a typical North Sea oil [11]. In this chapter, an introduction to bitumen structure and properties, and a short review of the published literature on PmB is provided. It can be assumed, therefore, as constant for heavy fractions to evaluate the internal consistency of measured data, or to estimate missing information, as will be described in Section 6.3. It is used to refine crude oil. Petroleum is a mixture of several hydrocarbons. The longer the hydrocarbon chain, the higher the boiling point and the earlier on they drain from the column: We can replicate the fractional distillation of Petroleum in the school laboratory, by using specialist glassware and a synthetic Crude Oil Substitute made up to a recipe suggested by CLEAPSS. Question 5: Name two places in India where coal is found. However, it is brittle in cold environments and softens readily in warm environments. The molecular weight, however, is expected to increase with increasing carbon number. In order to use petroleum oil as a good lubricant, some additives must be added to obtain certain desirable properties. CNG is considered to be a cleaner gas because it causes less pollution as compared to other fuels like petrol or diesel. Put the synthetic crude oil in the flask and attach the fractionating column and condenser as shown above. This is supplied to the top of the column at the boiling temperature and is variable in composition. By using this site you agree to these cookies being set. However, detailed information on the content of each SCN group may be required in special cases, e.g., when two hydrocarbon liquid phases or liquid-solid hydrocarbons are formed. Definition, Types, Complexity, Examples, Types of Quadrilaterals - Rectangle, Square, Rhombus, Parallelogram | Class 8 Maths. Finally, an outline of the focus and content of each chapter in the book is described.  The above equation becomes less reliable at M>300. But before that, we should be familiar with the basic term i.e fossil fuels. The boiled-off fractions are collected as distillates, each within a temperature band at the column top. Question 2: Define the term petrochemicals.
The above equation becomes less reliable at M>300. But before that, we should be familiar with the basic term i.e fossil fuels. The boiled-off fractions are collected as distillates, each within a temperature band at the column top. Question 2: Define the term petrochemicals.  The standard method is fully described in ASTM 2892-84 [2]. Natural gas is found above petroleum, trapped between the rock cap & petroleum layer. Figure 13.15. Because the receiver does not have to be removed and replaced during the distillation process, this type of apparatus is useful when distilling under an inert atmosphere for air-sensitive chemicals or at reduced pressure. [6], The fractional distillation of organic substances played an important role in the 9th-century works attributed to the Islamic alchemist Jabir ibn Hayyan, as for example in the Kitb al-Sabn ('The Book of Seventy'), translated into Latin by Gerard of Cremona (c. 11141187) under the title Liber de septuaginta. No single oil possesses all the properties needed to be a good lubricant. - Occurrence, Refining, Formation, Uses. As the distance from the still pot increases, a temperature gradient is formed in the column; it is coolest at the top and hottest at the bottom. Balasubramanian Viswanathan, in Energy Sources, 2017. The two major types of distillation columns used are tray and packing columns. This gas is a cause of acid rain. To separate the different fractions of petroleum from this impure petroleum reserve, fractional distillation is performed. Methods, relying on material balance and empirical correlations, have been proposed [4,13] to estimate the PNA content, using the specific gravity and molecular weight of each fraction. The measured properties are used in some generalised correlations, Section 6.2, to determine the critical properties and the acentric factors.
The standard method is fully described in ASTM 2892-84 [2]. Natural gas is found above petroleum, trapped between the rock cap & petroleum layer. Figure 13.15. Because the receiver does not have to be removed and replaced during the distillation process, this type of apparatus is useful when distilling under an inert atmosphere for air-sensitive chemicals or at reduced pressure. [6], The fractional distillation of organic substances played an important role in the 9th-century works attributed to the Islamic alchemist Jabir ibn Hayyan, as for example in the Kitb al-Sabn ('The Book of Seventy'), translated into Latin by Gerard of Cremona (c. 11141187) under the title Liber de septuaginta. No single oil possesses all the properties needed to be a good lubricant. - Occurrence, Refining, Formation, Uses. As the distance from the still pot increases, a temperature gradient is formed in the column; it is coolest at the top and hottest at the bottom. Balasubramanian Viswanathan, in Energy Sources, 2017. The two major types of distillation columns used are tray and packing columns. This gas is a cause of acid rain. To separate the different fractions of petroleum from this impure petroleum reserve, fractional distillation is performed. Methods, relying on material balance and empirical correlations, have been proposed [4,13] to estimate the PNA content, using the specific gravity and molecular weight of each fraction. The measured properties are used in some generalised correlations, Section 6.2, to determine the critical properties and the acentric factors.
- Polywood Lumber Suppliers
- Studio Mcgee Wall Sconce Target
- Matte Black Drop-in Kitchen Sink
- Chemical Guys Ceramic Coating Maintenance
- Ulanzi Vl61 Rgb Fill Light
- Hyatt House Cobb Galleria
- Humble Bee Ventilated Suit

fractional distillation of petroleum